
These features, along with their low cost, make them attractive for use in motor vehicles to provide the high current required by starter motors.
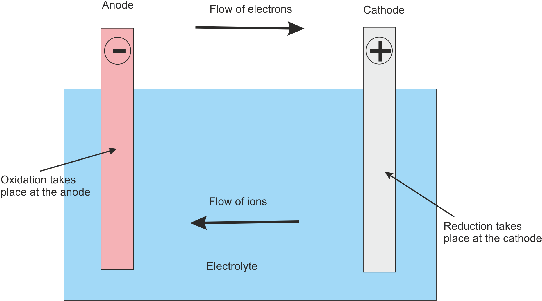
Despite this, their ability to supply high surge currents means that the cells have a relatively large power-to-weight ratio. Compared to modern rechargeable batteries, lead–acid batteries have relatively low energy density. It is the first type of rechargeable battery ever created. The aging of lead acid batteries is mainly caused by internal corrosion of the lead structure of the electrodes, the formation of fine short circuits, and by sulfating of the lead.The lead–acid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Planté. The power rating when new is not directly affected by this, as a weaker lead structure can also be designed with a large active surface area. When lead acid batteries are of the same capacity and size, but of different weights, the heavier battery usually lasts longer because the lead frames are stronger. But these batteries differ significantly from car batteries. Drive batteries or storage batteries can achieve a lifetime of between 5 and 15 years, depending on quality and stress. Especially in cars as starter battery, this is often insufficiently accurate, so that only 2 to 4 years of use are typical. However, voltage regulation is crucial here. Lead-acid batteries can achieve quite a long lifetime of several years. Lead acid batteries can deliver high currents for short periods and have a high power density. The nominal voltage of a cell is about 2V, but the voltage varies between approximately 1.75 V and 2.4V depending on the SoC and the charging or discharging current. This membrane also prevents electrical shorting through the electrolyte. In case the electrodes come into contact with each other through the physical movement of the battery or changes in the thickness of the electrodes, an electrically insulating but chemically permeable membrane separates the two electrodes. Both electrodes are immersed in an electrolytic solution of sulfuric acid and water. The positive electrode consists of lead oxide. The lead is porous to facilitate the formation and dissolution of lead. Composition of Lead-acid BatteryĪ lead-acid battery consists of a negative electrode made of spongy or porous lead. Industrial fields of applications for lead acid batteries are as traction power for mining vehicles, forklifts and as stationary power sources such as emergency back up power storage (UPS) and signaling stations for railroads and telecommunication. In 1992 about 3 million tons of lead were used in the manufacture of batteries. Most of the world’s lead–acid batteries are automobile starting, lighting, and ignition (SLI) batteries, with an estimated 320 million units shipped in 1999. Its low manufacturing cost and its high surge current levels make it common where its capacity (over approximately 10 Ah) is more important than weight and handling issues. The disadvantage of this battery chemistry is that it is very sensitive to deep cycling compared to other battery systems, and due to the high density of lead, the specific energy of the batteries is quite low.

The lead–acid battery is relatively heavy for the amount of electrical energy it can supply. The lead sulfate first forms in a finely divided, amorphous state and easily reverts to lead, lead dioxide, and sulfuric acid when the battery recharges. Lead and lead dioxide, the active materials on the battery’s plates, react with sulfuric acid in the electrolyte to form lead sulfate. Lead acid batteries typically have coulombic efficiencies of 85% and energy efficiencies in the order of 70%. The battery contains liquid electrolyte in an unsealed container, requiring it to be kept upright and the area well ventilated to ensure safe dispersal of the hydrogen gas it produces during overcharging. Lead-acid batteries are secondary (rechargeable) batteries that consist of a housing, two lead plates or groups of plates, one of them serving as a positive electrode and the other as a negative electrode, and a filling of 37% sulfuric acid (H 2SO 4) as electrolyte.


 0 kommentar(er)
0 kommentar(er)
